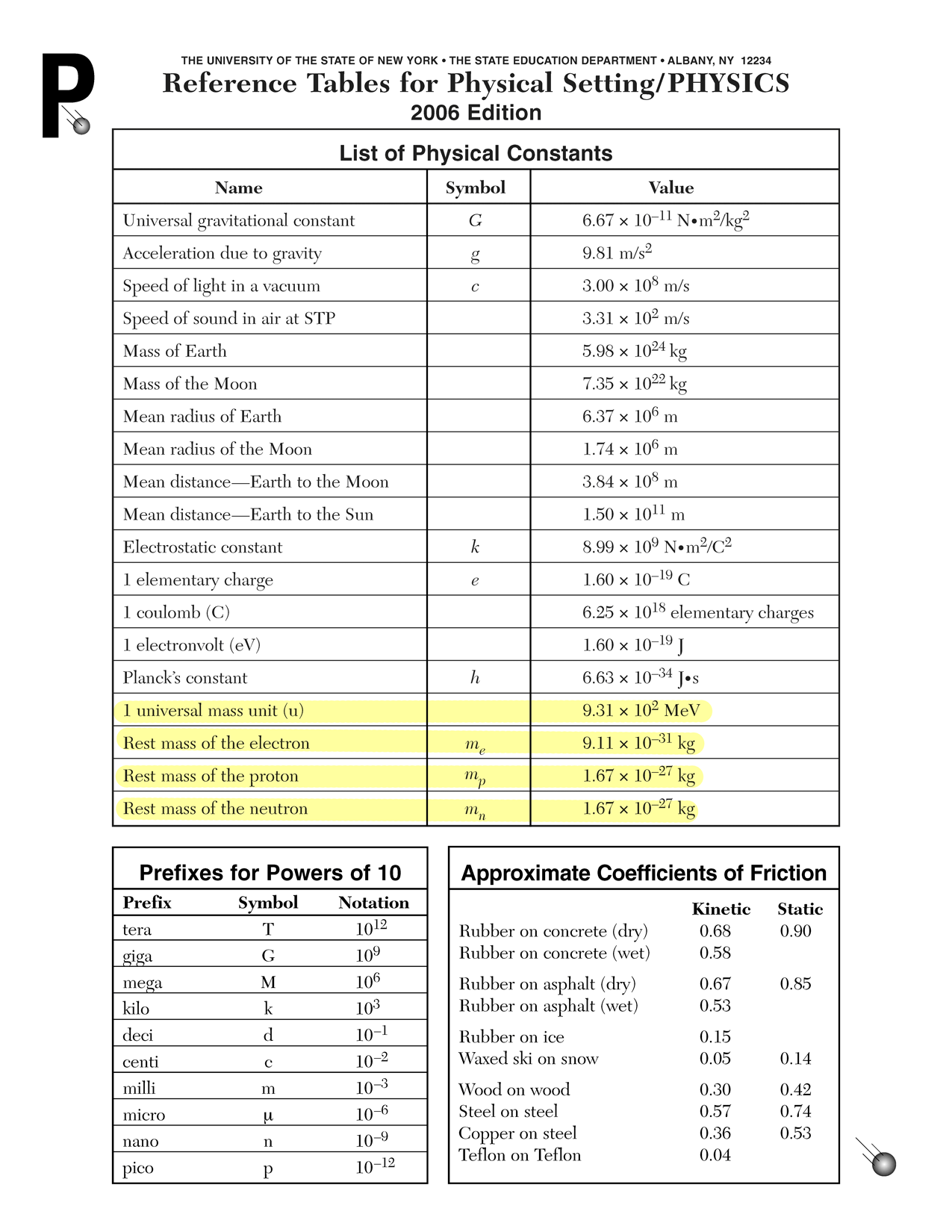
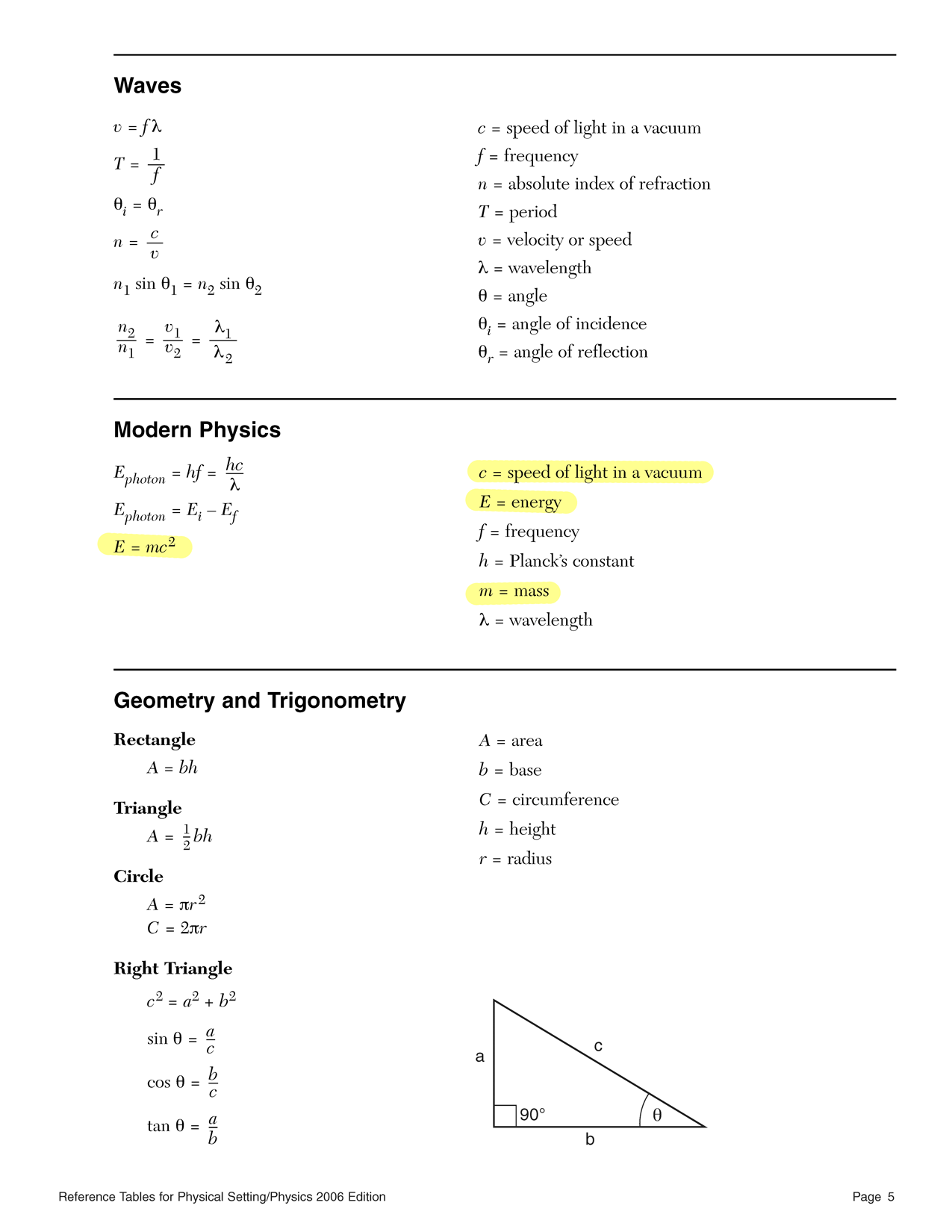
Physics A: Problem Set 26: Mass-energy
recommended reading
| High Marks: | 5:30–5:36 |
| Barron's Let's Review: | 13.6 Energy and mass |
| physics.info: | n/a |
| Wikipedia: | Mass-energy equivalence, Nuclear reaction, Pair production, Annihilation, Antimatter |
| HyperPhysics: | Relativistic energy |
| Khan Academy: | Nuclear chemistry (the first four chapters) |
| Mr. Machado: | 03 Mass-Energy Equivalence |
sample problem
- The fuel used in most high-yield thermonuclear weapons is solid lithium 6 deuteride. These weapons, commonly known as "hydrogen bombs" or "H-bombs", use the energy released when a nucleus of light lithium, also known as lithium 6 (63Li, m = 6.015121 u), and heavy hydrogen, also known as deuterium (21H, m = 2.0140 u), fuse to form two nuclei of ordinary helium (42He, m = 4.00260 u).
- Write this reaction out in symbolic form.
- What is the mass defect when one molecule of lithium 6 deuteride is transformed into two atoms of helium? State your answer in…
- atomic mass units
- megaelectronvolts
- joules
- kilograms
Here's the reaction in symbolic form.
63Li + 21H → 2 42He
Most of the rest of this problem is an exercise in conversion — conversion between mass and energy in SI units and acceptable non-SI units.
Mass before does not equal mass after. The parents weigh more than the daughters in this reaction. This means that the reaction releases energy.
+ 6.015121 u + 2.014000 u + 8.029121 u + 4.00260 u + 4.00260 u + 8.00520 u ∆m = 0.023921 u
Convert atomic mass units (u) to megaelectronvolts (MeV) using the conversion factor.
0.023921 u × 931 MeV/u Convert megaelectronvolts to joules using the conversion factor better known as the elementary charge. Remember that mega means 106.
22.27 × 106 V × 1.60 × 10−19 C Convert joules to kilograms using Einstein's (rearranged) equation.
m = E c2 m = (3.563 × 10−12 J) (3.00 × 108 m/s)2
homework
- A standard measure of explosive energy is the ton of TNT. By definition, one ton of TNT possesses 4.184 GJ of chemical energy.
- The "Little Boy" uranium fission bomb dropped on Hiroshima, Japan on 6 August 1945 had an explosive yield of 12.5 kilotons of TNT. How much mass was transformed into energy by this weapon? About how big is this?
- The nuclear weapon with the greatest yield was the 50 megaton, two-stage fusion "Tsar Bomba" (Цар Бомба) detonated over Novaya Zemlya, Russia on 30 October 1961. How much mass was transformed into energy by this weapon? About how big is this?
Use the famous equation…
E = mc2
and solve it for mass
m = E c2 The kilo in kilotons means 103.
m = E c2 m = (12.5 × 103)(4.184 × 109 J) (3.00 × 108 m/s)2 When the Little Boy exploded, it destroyed about half a gram — the mass of a paperclip.
The mega in megatons means 106.
m = E c2 m = (50 × 106)(4.184 × 109 J) (3.00 × 108 m/s)2 The Tsar of bombs (or is it the bomb of the Tsar) destroyed the mass of a small cat, or a big chicken.
- When four hydrogen nuclei fuse to form one helium nucleus (plus two electrons and two antineutrinos) 0.02870 u (universal mass units) are converted into energy.
- What is this energy in megaelectronvolts?
- What is this energy in joules?
- What is this mass in kilograms?
This is a question mostly about using the reference tables for this class— in particular, the list of physical constants on the front.
According to the reference tables…
1 u = 9.31 × 102 MeV
Therefore…
0.02870 u × 931 MeV/u According to the reference tables…
1 eV = 1.60 × 10−19 J
and…
Prefix Symbol Notation mega M 106 Therefore…
26.72 × 106 eV × 1.60 × 10−19 J/eV Now we need an equation — the equation.
E = mc2
Solve it for mass.
m = E c2 Numbers in.
m = 4.275 × 10−12 J (3.00 × 108 m/s)2 Answer out.
m = 4.750 × 10−29 kg
- Neutron induced fission is the class of nuclear reaction powering nuclear reactors and nuclear weapons. A particular isotope of uranium or plutonium is bombarded with a bullet neutron. The now slightly heavier nucleus is unstable and splits (fissions) into two roughly equal halves plus 2–3 free neutrons. For each of the incomplete reactions below, determine the number of free neutrons.
- 10n + 23592U → 8735Br + 14657La + …
- 10n + 23592U → 9236Kr + 14156Ba + …
- 10n + 23592U → 9037Rb + 14455Cs + …
- 10n + 23592U → 9038Sr + 14354Xe + …
- 10n + 23994Pu → 9436Kr + 14458Ce + …
Check the mass numbers on both sides of the reaction. The totals should be the same, but they aren't because the reaction was deliberately written as incomplete. The number you're short on the daughters' side (the right side) is the number of neutrons released.
- 10n + 23592U → 8735Br + 14657La + 3 10n
- 10n + 23592U → 9236Kr + 14156Ba + 3 10n
- 10n + 23592U → 9037Rb + 14455Cs + 2 10n
- 10n + 23592U → 9038Sr + 14354Xe + 310n
- 10n + 23994Pu → 9436Kr + 14458Ce + 2 10n