Half Life
Practice
practice problem 1
solution
practice problem 2
| mass number | abundance (%) | half life (years) |
|---|---|---|
| 235 | 0.7201 | 7.04 × 108 |
| 238 | 99.275 | 4.46 × 109 |
Determine the age of the Earth using Rutherford's method and contemporary data, then compare your results with the currently accepted value of 4.55 × 109 years and discuss.
solution
Divide the half life equation by itself. Give the numerator to the lighter isotope and the denominator to the heavier isotope. Plug in the appropriate values.
| N(235) | = | N02−t/T½(235) |
| N(238) | N02−t/T½(238) | |
| 00.7201% | = | 2−t/7.04 × 108 yr |
| 99.275% | 2−t/4.46 × 109 yr | |
| 0.00725 | = | 2−t/7.04 × 108 yr |
| 2−t/4.46 × 109 yr |
Normally, I'd reduce this equation down until the unknown quantity (t) was on the left side of the equals sign and everything else was on the right side. I don't recommend that in this case. You'll wind up with a base 2 log function in the results. I don't own a calculator with that capability built in. However, I do own a calculator that can draw graphs and allows me to trace over the curve, testing the value at various spots.
Plot this function (or something similar looking).

Set an appropriate range for the displayed window. I suspect the answer should be in the billions, so I'll set the horizontal range from 0 to 10 billion. Draw the graph and then probe it to find values around 0.00725.
 |
 |
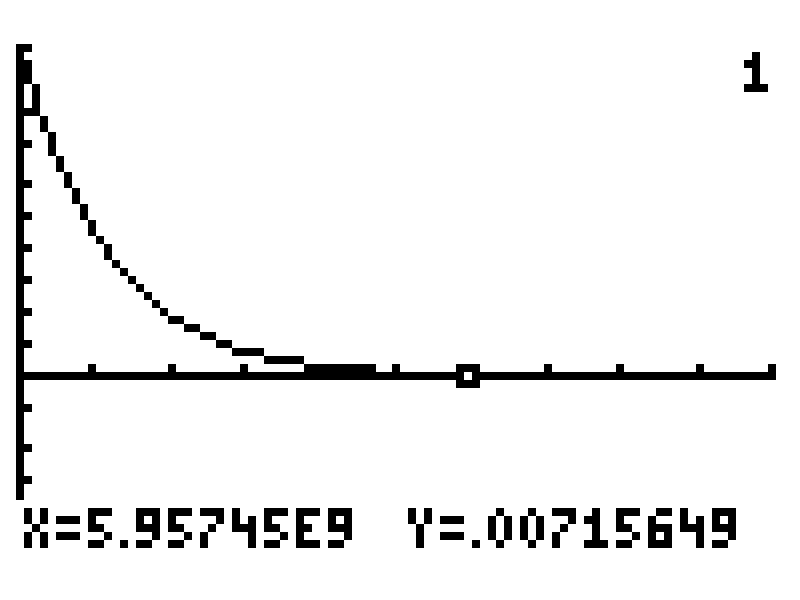 |
Those values are pretty close. Let's zoom in more to see if we can land on a value closer to our goal of 0.00725.
 |
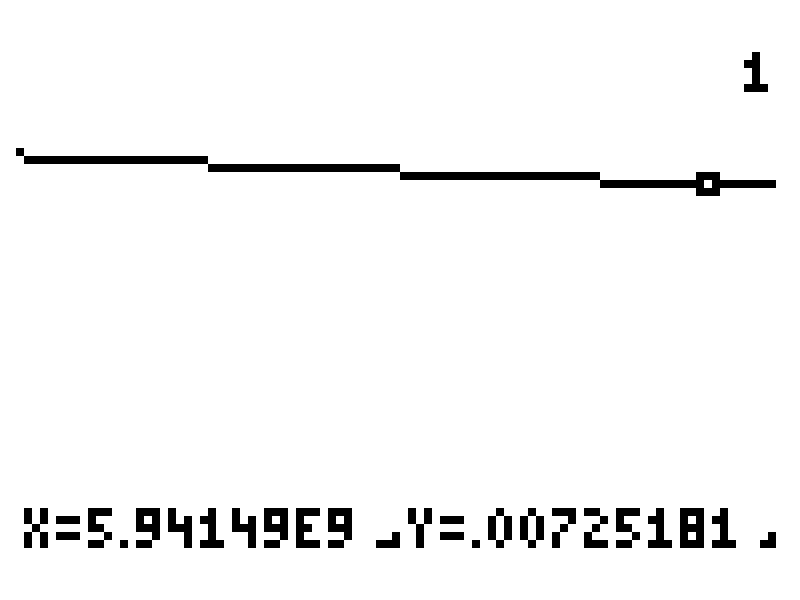 |
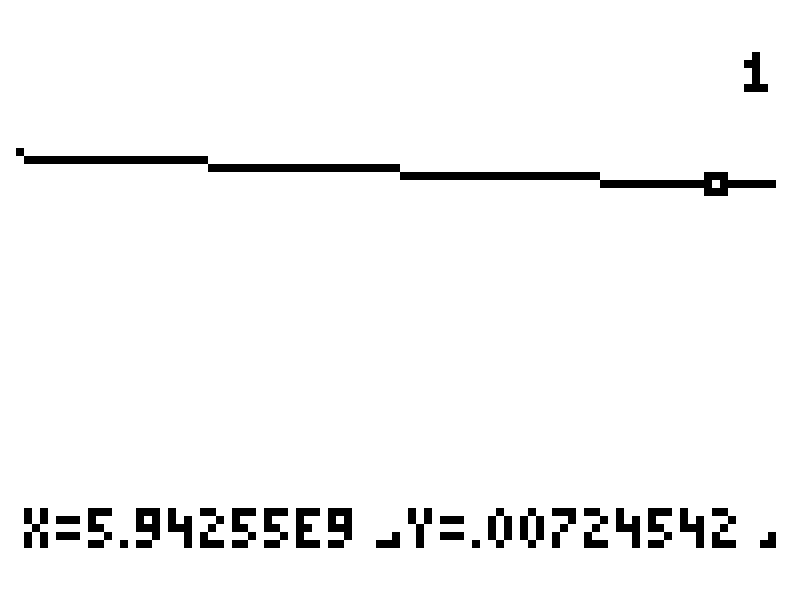 |
Note how the curve looks like a straight line at this resolution. We've zoomed in quite a bit. The values to the left and right are converging towards our goal and are in agreement to within 3 significant digits. I think we've found our solution.
t = 5.94 × 109 years
You can also use an online calculator if you wish.
0.7201/99.275=2^(-t/7.04e8)/2^(-t/4.46e9)
You get the same answer.
t = 5.94 × 109 years
This result is…
| ∆t | = |
|
||
| taccepted | ||||
| ∆t | = 31% too large | |||
| taccepted | ||||
This method is only good enough to give an order of magnitude estimate of the age of the Earth. The problem lies in the assumption that the two main isotopes of uranium ever had equal abundances. This is not the case.
The sun was formed from the residue of stars that went supernova. We know that it is a second generation star because of the presence of heavy elements. The only way to naturally produce nuclei heavier than iron is in a supernova. This process was first described in a 1957 paper that is usually known by the last names of the authors in alphabetical order — Burbidge, Burbidge, Fowler, and Hoyle. The abundance of all the elements heavier than hydrogen and helium can be calculated by the processes described in this paper. In the case of uranium, supernovas always produce more 238U than 235U.
Rutherford devised another method for determining the age of the Earth in the same year as the one described in this problem. This second method gave him a value of 3 billion years, which is 34% too small. The accepted value of 4.55 billion years quoted in this problem was determined by Claire Patterson (1922–1995) at the California Institute of Technology in 1956. It remains the definitive value to this day.
practice problem 3

The SNAP-27 activated by the Apollo XIV crew on 5 February 1971 used 3.8 kg of plutonium 238 dioxide and generated 73 W of power when first turned on. If 238PuO2 has a half life of 87.74 years and decays via the emission of 5.593 MeV alpha particles, determine…
- the initial power radiated by the plutonium fuel
- the initial efficiency of the generator
- the power of the generator on the anniversary of the Apollo XIV mission from the day it was turned on until its centennial.
solution
Let's begin by determining the number of atoms in 3.8 kg of plutonium 238 dioxide. Recall that oxygen has an atomic mass of 16 and therefore that 238PuO2 has a molecular mass of 238 + 2(16) = 270.
N0 = (3800 g)(6.02 × 1023 atom/mol) (270 g/mol) N0 = 8.47 × 1024 plutonium atoms Half of this will have decayed after one half life giving an average activity of…
A = ½N0 T½ A = ½(8.47 × 1024 decay) (87.74 yr)(365.25 × 24 × 60 × 60 s/yr) A = 1.53 × 1015 Bq This is an average value, but we want the initial value. If the activity decreased linearly, the average value would be the average of the initial and final values. But this is radioactivity and radioactivity goes down exponentially not linearly. We will have to resort to calculus for this next step. The average value of a varying quantity is its definite integral divided by the interval.
A = 1 ⌠
⎮
⌡A dt T½ T½ A = 1 ⌠
⎮
⌡A02−t/T½ dt T½ 0 A = 1 A0T½ T½ 2 ln 2 A = A0 2 ln 2 Rearrange to make the initial activity the subject of the equation and substitute numbers for variables.
A0 = 2 ln 2 A
A0 = 2(ln 2)(1.53 × 1015 Bq)
A0 = 2.12 × 1015 BqMultiply this by the energy per decay (in joules) to get the power (in watts).
P0 = A0E
P0 = (2.12 × 1015 decay/s)(5.593 × 106 eV/decay)(1.60 × 10−19 J/eV)
P0 = 1,900 WEfficiency is the ratio of energy output to energy input.
η = Eout Ein η = 73 J/s 1,900 J/s η = 3.8% Not very efficient, is it?
Assume the power output decays in the same manner as the activity, use years as the unit of time, and generate a formula for a spreadsheet or data analysis application.
P = P02−t/T½
P = 73 W × 2(1971 − t[yr])/(87.74 yr)The results are listed in the text file snap.txt.