Engines
Problems
practice
- 0.40 moles of an ideal, monatomic gas runs through a four step cycle. All processes are either adiabatic or isochoric. The pressure and volume of the gas at the extreme points in the cycle are given in the table below.
- Sketch the PV graph of this cycle.
- Determine the temperature at state A, B, C, and D.
- Calculate W, Q, and ΔU on the path A→B, B→C, C→D, D→A and for one complete cycle. (Include the algebraic sign with each value.)
- For an engine running this cycle, determine its…
- real efficiency
- ideal efficiency
A four step cycle (adiabatic & isochoric) state A B C D P (Pa) 100, 000 1,462, 000 5,850, 000 400, 000 V (m3) 0.010 0.002 0.002 0.010 T (K) path A→B B→C C→D D→A ABCDA description adiabatic isochoric adiabatic isochoric closed cycle ΔU (J) Q (J) W (J) - Write something completely different.
- Write something different.
- Write something completely different.
conceptual
- If your goal is to improve the theoretical efficiency of an engine, is it better to increase the temperature of the hot reservoir by a certain amount or decrease the temperature of the cold reservoir by the same amount? Justify your answer with calculations.
numerical
- A series of 4 connected questions about a human heart.
- A healthy adult heart pumps 80 mL of blood per contraction and contracts once each second. Blood pressure within the circulatory system varies from a maximum (systole) of 16 kPa (120 torr) to a minimum (diastole) of 10.7 kPa (80 torr). Determine the average power generated by a human heart.
- The heart actively works during one-third of each cycle and rests for the remaining two-thirds of the cycle. Determine the power generated by a human heart during the pumping phase.
- The mechanical efficiency of the heart is about 9% (only 9% of the energy it consumes goes to actual work). Determine the average power consumed by a human heart.
- During strenuous exercise, the heart pumps 5 times more blood per minute and blood pressure increases by 50%. Determine the average power consumed by an exercising human heart.
- The largest piston engines in the world are used to propel container ships. Some data for one of these behemoths is given in the table below.
Wärtsilä RTA96C (14 cylinder model) specification value displacement 25.48 cubic meters power 80.08 megawatts torque 7.604 meganewton meters rotational speed 102 rotations per minute fuel consumption 13,690 liters per hour fuel energy density 42.70 megajoules per liter peak pressure 14.5 megapascals - Calculate the following quantities in gigajoules per hour…
- the heat produced by burning fuel
- the useful work done by the engine
- the heat exhausted to the environment
- What is the real efficiency of this engine?
- Calculate the following quantities in gigajoules per hour…
- Ocean Thermal Energy Conversion (OTEC) is a proposed method for extracting energy by exploiting the temperature difference between warm surface water and cold deep water in the ocean. A heat engine would have one side connected to a pipe drawing water from the surface and the other side connected to a pipe drawing water from a thousand meters deep. The engine would drive a generator that would produce electricity. The ideal place for siting such a power facility would be in the tropics (or near tropics) where surface waters can be as hot as 25 °C and deep water as cold as 5 °C.
- Determine the maximum theoretical efficiency of an OTEC heat engine.
- At what rate would heat have to be extracted from the surface of the ocean to equal the power output of a 1.25 GW natural gas-fired, steam turbine driven power plant?
- What advantages does an OTEC system offer over a facility like the one described in the previous part of this problem?
- What disadvantages have kept OTEC systems from being accepted for large scale, commercial power generation?
- An ideal gas is run through a closed cycle ABCA. The cycle starts at state A (V = 0.004 m3, P = 100 kPa, T = 800 K). The gas is compressed isobarically to state B where it has half its initial volume. It is then heated isochorically to state C where it has twice its initial pressure. Finally, it returns to state A along a straight line path on a PV graph.
- How many moles of gas are involved in this process?
- Determine the pressure, volume, and temperature of state B.
- Determine the pressure, volume, and temperature of state C.
- Sketch the PV graph of the cycle ABCA.
- Determine the net work done by the gas after one cycle. Include the algebraic sign in your answer. State whether the net work was done by the gas on the environment or on the gas by the environment.
- Determine the net heat transfered to/from the gas after one cycle. Include the algebraic sign in your answer. State whether the net heat transfer was into or out of the gas.
- Determine the ideal efficiency of the cycle.
- Extra credit: Determine the real efficiency of the cycle.
- 0.040 moles of an ideal, monatomic gas runs through a three step cycle. All processes follow straight line paths on a pressure-volume graph. The pressure and volume of the gas at the extreme points in the cycle are given in the first two rows of the table below.
- Sketch the PV graph of this cycle.
- Determine the temperature at state A, B, and C.
- Calculate W, Q, and ΔU on the path A→B, B→C, C→A and for one complete cycle. (Include the algebraic sign with each value.)
- For an "engine" running this cycle, determine its…
- real efficiency
- ideal efficiency
A three step cycle (straight line processes) state A B C P (kPa) 100 100 200 V (m3) 0.002 0.001 0.001 T (K) path A→B B→C C→A ABCA description isobaric isochoric diagonal closed cycle ΔU (J) Q (J) W (J)
statistical
-
The United States in a humongous engine. Energy comes in, energy goes out, and work is done. Every year since 1976 (and a for a few years before that), the Lawrence Livermore National Laboratory (LLNL) has released annual flowcharts that illustrate the production and consumption of energy for the whole of the United States. Primary energy sources are on the far left (petroleum, natural gas, coal, nuclear, biomass, wind, hydro, solar, geothermal) Energy consumption sectors are on the near right (transportation, industrial, residential, commercial) with electricity generation as an intermediate step. Ultimately, all energy winds up on the far right as useful energy (energy services) or rejected energy (lost energy).
The images below link to a copy of every LLNL energy flowchart I could find. Use the information in these flowcharts to create four time series graphs for the US machine and comment briefly on each one. The overall trend in one graph may shock you.

1950 
1960 
1970 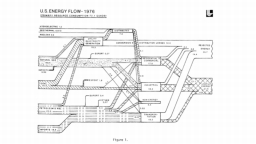
1976 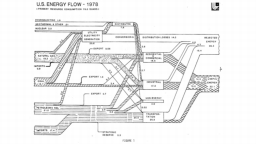
1978 
1979 
1980 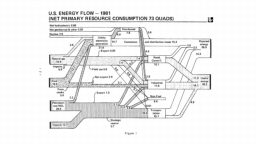
1981 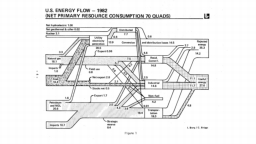
1982 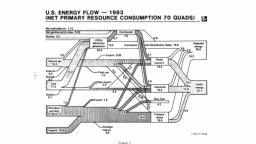
1983 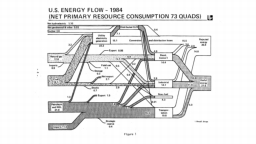
1984 
1985 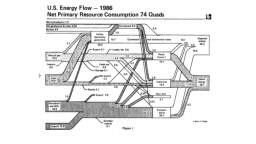
1986 
1987 
1988 
1989 
1990 
1991 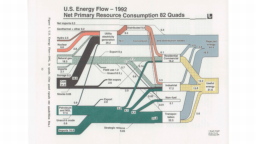
1992 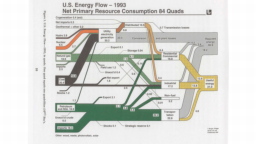
1993 
1994 
1995 
1996 
1997 
1998* 
1999 
2000 
2001 
2002 
2003 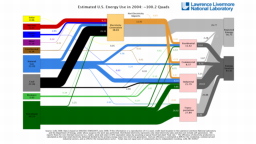
2004 
2005 
2006 
2007 
2008 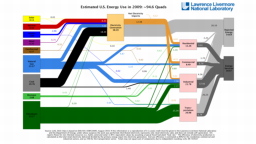
2009 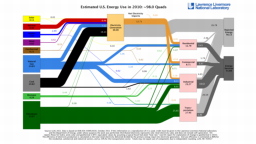
2010 
2011 
2012 
2013 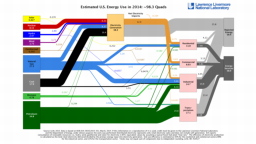
2014 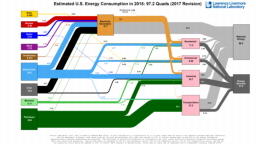
2015 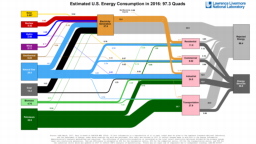
2016 
2017 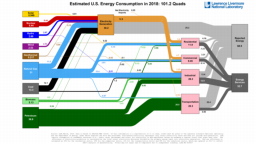
2018 
2019 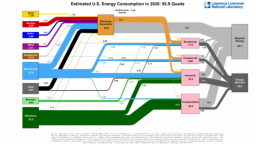
2020 
2021 
2022 
2023 *The flowchart for 1998 has values in exajoules. All other flowcharts are in quads.Some notes for people who like to know stuff. First, although LLNC produces these diagrams, the data used to produce them comes from the US Energy Information Agency (EIA). In a sense, the EIA provides the statisticians and the LLNC provides the artists. Second, the EIA is an American institution and America still has a ways to go when it comes to the International System of Units. Energies are reported in quads instead of joules or multiples of a joule. A quad is a quadrillion British thermal units (Btu). A Btu is the energy needed to raise one pound of water by one degree Fahrenheit (°F) and a quadrillion is one followed by 15 zeros (1015). For comparison: US gallon of gasoline has about 100,000 Btu (105 Btu) of chemical energy, and a US toaster uses about 100 Btu (102 Btu) of electrical energy in the two minutes it takes to toast two slices of bread. For simplicity sake, a quad is almost the same as an exajoule (1018 J).1 quad = 1.05505585262 × 1018 J
The choice of units doesn't matter since this is a question about trends. If you have an aversion to non-SI units, then replace every value in quads with its equivalent in exajoules. If you're OK with quads, then replace every value in exajoules with its equivalent in quads.
(exact by definition)- energy consumption vs. year
- useful energy vs. year
- rejected energy vs. year
- efficiency vs. year